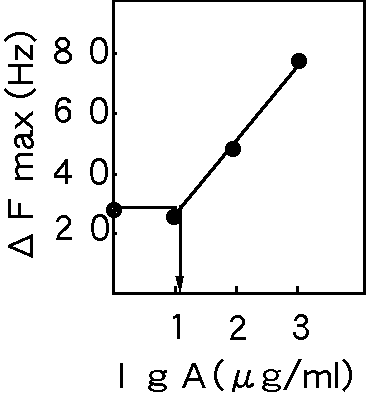A piezoelectric quartz crystal immunosensor has been developed for the
detection of antibodies in human saliva. The system consists of the quartz
piezoellectric crystal, oscillator and frequency counter. The antibodies
against human immunoglobulin A are immobilized onto the gold electrode
surface on the quartz crystal. Immobilization techniques used in this work
are adsorption method and covalent bond method. The latter is more stable
in repeatitive assay. Immunoreaction of the antibodies in diluted human
saliva is monitored using quartz crystal microbalance biosensor.
Keywords : Saliva ; Antibody ; Immunoglobulin ; Quartz ; biosensor
1. Introduction
The linear relationship between the frequency change of an AT-cut
piezoelectric quartz crystal and the mass deposited on the crystal [1] is very useful for
micro-gravimetric immunosensor [ 2, 3
] and DNAsensor [ 4, 5 ]. Human immunoglobulin A ( IgA
) in saliva in the range of several hundred microgram quantities has been
detected with method of enzyme-linked immunosorbent assay ( ELISA ). The
method using quartz crystal microbalance is simple in direct
detection of immunoreaction in real time but with low repeatability.
In this study, immobilization of the antibodies against human
immunoglobulin A ( anti-IgA ) onto the electrodes surface is examined and
immunoreaction between anti-IgA and antibodies in diluted human saliva is
monitored with quartz crystal microbalance biosensor.
2. Experimental
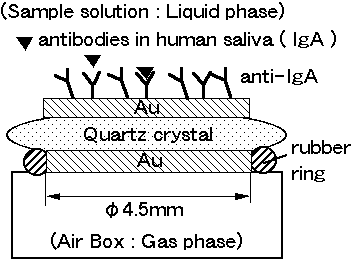
Figure 1. Structure of
quartz crystal microbalance biosensor and antibodies.
Figure
1 shows the structure of quartz crystal microbalance biosensor. Mouse
monoclonal antibodies against human IgA were immobilized on gold electrode
of quatz crystal microbalance. Immobilization methods of anti-IgA were
adsorption or the covalent bonding onto the surface of the crystal.
Immobilization method by adsorption was used of solution of
anti-IgA ( 2 mg Bio-ZyMED Co. ) in 10 mmol sodium acetate buffer. The
solution ( 20 microliter ) was dropped on the one side ( liquid phase ) of a quartz crystal
and stand for 24 hours at 37 centigrade. After the solution removed, the
immobilized electrode surface with anti-IgA was washed with phosphate
buffer solution ( PBS pH 7.4 ).
Immobilization method by covalent bond
was carried out by reaction between gold and cysteamine. After reaction of
electrode (Au) and cysteamine ethanol solution ( 1 mmol, 11.3 mg / 100 ml
pH 6.4 ) for 1 hour at room temperature, 15 microliter of anti-IgA solution
( 1 mg/ml ) and 5 microliter of 1-ethyl-3-( 3-dimethylaminopropyl
)carbodiimiide HCl solution ( 5 mg/ml) in sodium acetate were mixed and
standed for 24 hours at 4 centigrade.
One side of quartz crystal was set on the O-ring sealed air box. On the other side of the quartz crystal for sample liquid, anti-IgA was immobilized.
Quartz crystal was oscillated between sample solution ( liquid phase ) and air box
( gas phase ).
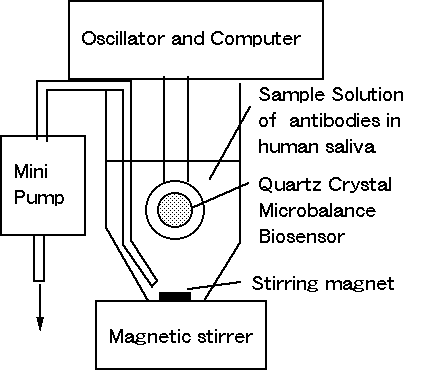
Figure 2. Monitor system of
antibodies in human saliva.
Figure 2 shows the measuring apparatus for immunoreaction between
immobilized anti-immunoglobulin and immunoglobulin in sample solution.
Immobilized quartz crystal was oscillated by oscillator ( Sogo
Pharmaceutical Co. Ltd : SF-105 ) in basic frequency 9 MHz. The
oscillation frequency was counted and fed into computer ( Biopac System
INC.: MP100A-Macintosh 5300 and PC9800 ). Sample solution ( 10 ml ) with standard human IgA (Seikagaku Kogyo : IgA originated in human
saliva ) was prepared in the range of 1 to 3 micro g/ml with PBS or 100
time diluted human saliva with PBS. The sample solution was stirring and
wasted by bio-minipump. The dissociation buffer after immunoreaction was
used with 10 mmol HCl aquaeous solution.
3. Results and discussion
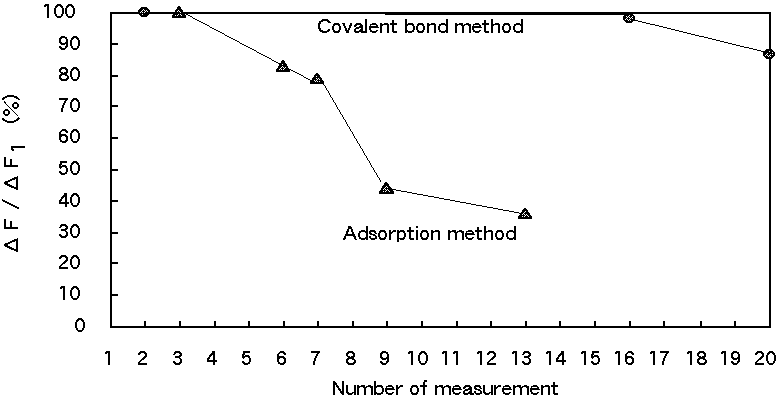
Figure 3. Repeatability on the frequency change (delta F) of quartz crystal microbalance biosensor immobilized with adsorption method or covalent bond method.
The first frequency change is delta F1.
Figure 3 shows the repeatability on the frequency change in immunoreaction by dissociation with HCl aquaeous solution. Immobilizaton
of anti-IgA onto the electrode surface on the quartz crystal by covalent
bond method has the much repeatability (more than 15 times in number of measurement) compared with that by adsorption method (only 3 times). It is considered that gold and sulfur covalent bonds formed by cysteamine maintain more stable immobilization of anti-IgA onto the electrode in repeatitive assay.
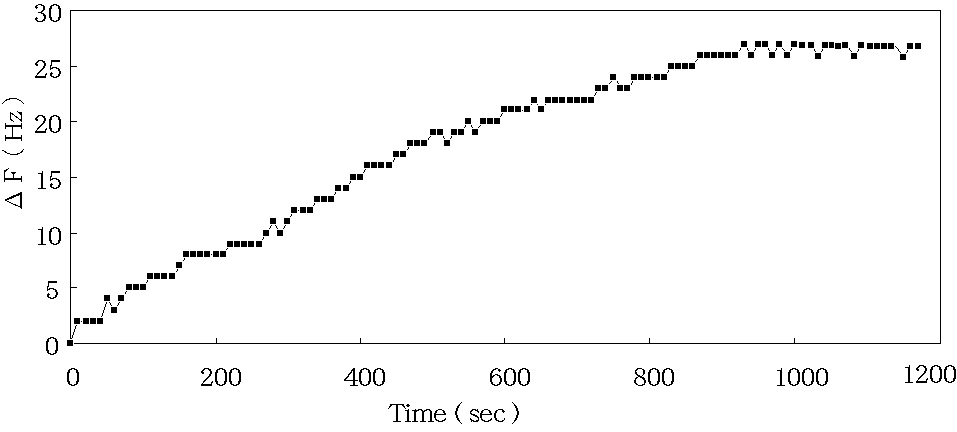
Figure 4. The frequency change of quartz crystal microbalance biosensor in diluted human saliva .
Figure 4 shows time course of the frequency change of the quartz crystal microbalance
biosensor immobilized anti-IgA onto electrode by covalent bond method.
Immunoreaction between the immobilized anti-IgA and 100 times diluted human saliva ( IgA ) is monitored by the frequency change in real time. The maximum frequency change is detected in about 1000sec.
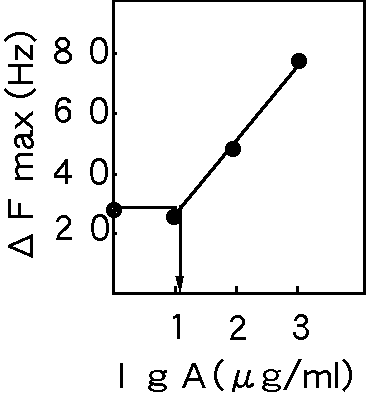
Figure 5. The relationship between the maximum frequency change of human saliva and the standard human IgA.
Figure 5 shows the relationship between the maximum frequency change of human saliva and the standard human IgA.
The frequency changes by standard human IgA were 26 Hz ( 1
micro g/ml ), 50 Hz ( 2 micro g/ml ) and 80 Hz
( 3 micro g/ml ). Antibodies in the one human saliva detected with ELISA method was 100
micro g/ml quantities . The 100 times diluted solution of this same human saliva was 27 Hz
at 1000 sec as shown in figure 4. This frequency change showed almost equivalent to 1 micro g/ml of standard human IgA. This result indicated 100 micro g/ml quantities of this human saliva with quartz crystal microbalance biosensor as same as ELISA method. From this example, the antibodies in human
saliva were monitored using quartz crystal microbalance biosensor.
4. References
[1]
G. Z. Sauerbrey, Z. Phys., 155 (1959) 206.
[2] G. Sakai, T. Saiki, T. Uda, N.
Miura and N. Yamazoe, Sensors and Actuators B, 24 (1995)
134.
[3] M. Muratsugu, F.
Ohta, Y. Miya, T. Hosokawa, S. Kurosawa, N. Kamo and H. Ikeda, Anal.
Chem., 65 (1993) 2933.
[4] Y. Okahata, Y. Matsunobu, K. Ijiro, M. Mukae,
A. Murakami and K. Makino, J. Am. Chem. Soc., 114 (1992)
8299.
[5] S. Yamaguchi, T.
Shimomura, T. Tatsuma and N. Oyama, Anal. Chem., 65(1993)
1925.
*Corresponding author
e-mail : ichiro@mosk.tytlabs.co.jp
Copyright ; CCAB97